Background:
The initial development work on microcellular foaming was done using a batch process. In this process, a plastic part is placed in a high pressure chamber and the chamber is filled with a supercritical fluid (shown in the figure below). The sample is typically left in the chamber sufficient time, normally in excess of 24 hours, to allow for saturation by the SCF. The saturation level is dependent on the pressure and temperature in the chamber. Carbon dioxide was the most common gas used by MIT.
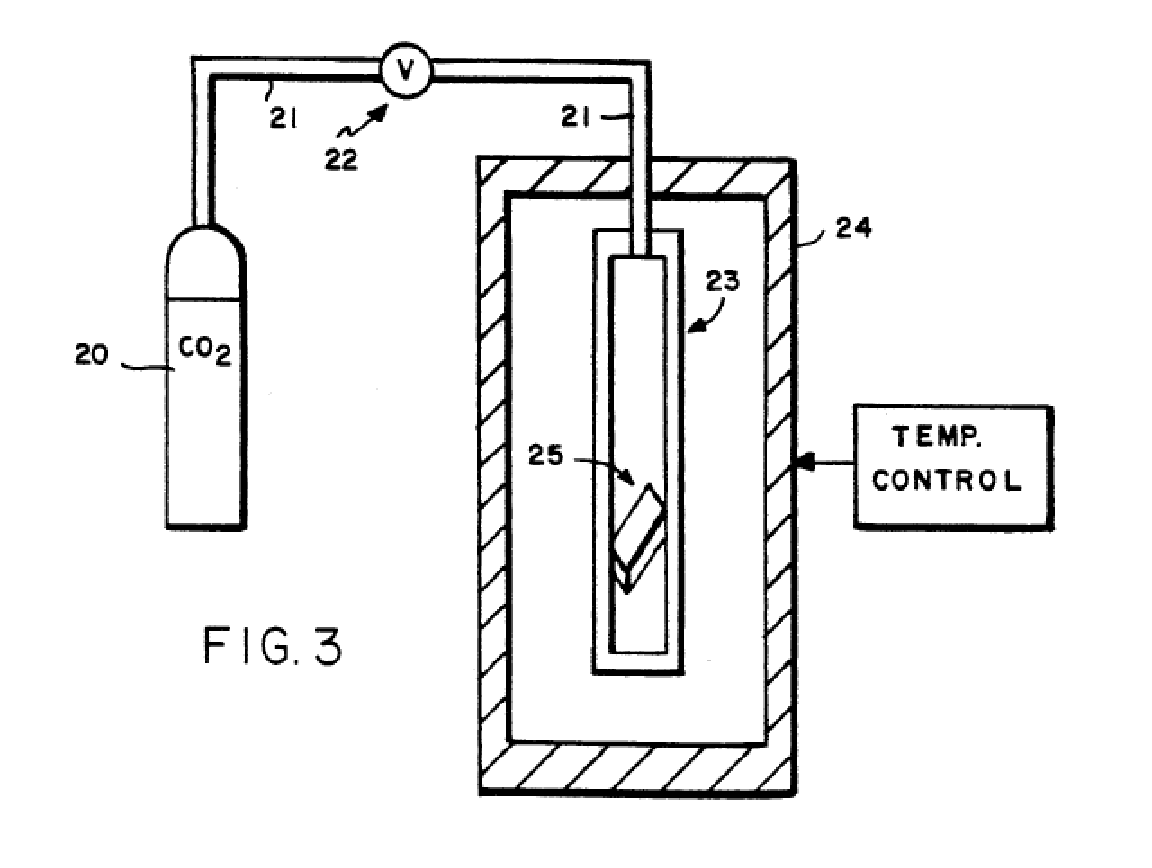
As the process continued to develop and transitioned from the batch process to extrusion, carbon dioxide continued to be the SCF of choice. Carbon dioxide was preferred to nitrogen in the extrusion process because the solubility level is much higher, which allowed for higher density reductions and also much lower process temperatures. In the extrusion process, the material foams at atmospheric pressure and the key control over cell growth is the temperature at which the material exits the die. Since the glass transition temperature and the crystallization temperature can be reduced with the amount of SCF in solution, the higher levels of carbon dioxide allowed for a greater reduction in these temperatures, particularly for amorphous materials and tandem extruders. As time has progressed and higher density foam solutions have become more popular i.e. those greater than 0,2g/cc, N2 has become more widespread for extrusion foaming. The reason being that N2 promotes smaller cell size for much lower weight percent of blowing agent thus reducing the need for specialized equipment to provide lower temperature, and handle undesired ascetics that result from high CO2 usage and expansion. Furthermore then reducing the need to tailor resin blends.
As the MuCell technology migrated to injection molding, nitrogen continued to becoame thea more common SCF. It is now the case that for injection molding, almost all customers use nitrogen.
Choosing an SCF:
There are key difference between nitrogen and carbon dioxide that result in very different behaviors in the foaming process is that carbon dioxide has a much higher solubility level than nitrogen. This basically means that more carbon dioxide can be put in solution but on injection into the mold cavity, the carbon dioxide tends to come out of solution more slowly.
As can be seen in Table 1, the estimated solubility of CO2 in the polymer is 3 to 5 times higher than that of nitrogen.
Table 1: Estimated Solubility Limits for Selected Polymers (200 C, 276 Bar)
| Carbon Dioxide | Nitrogen | |
| HDPE | 14% | 3% |
| PP Homopolymer | 11% | 4% |
| GPPS | 11% | 2% |
*Solubility limits are highly dependent on the temperature and pressure conditions.
A higher solubility level means that for a given set of molding conditions, barrel temperatures, back pressure and screw speed, more carbon dioxide can be dissolved into the polymer. The higher the level of SCF dissolved into the polymer, the greater the shift in the glass transition temperature and/or crystallization temperature and also the greater the reduction in polymer viscosity. While a reduction in glass transition temperature and/or crystallization temperature is important to foam extrusion processes, there is typically not sufficient residence time in the injection molding process forward of the SCF injection location to take advantage of the ability to reduce process temperatures. Therefore, the shift in this polymer characteristic is not typically a benefit for injection foam molding.
The shift in viscosity can be valuable. In most cases, it is possible to achieve up to twice the viscosity reduction with carbon dioxide as compared to nitrogen.
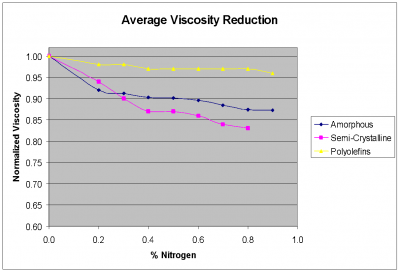
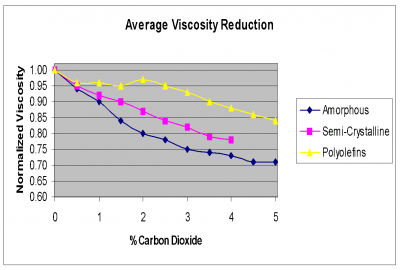
It should be noted that solubility limits assume an unlimited residence time. Typical exposure times of the polymer to the supercritical foaming agent in an injection molding process can be in the range of 1 minute up to 10 minutes. This limited time for the SCF to dissolve into the polymer make the actual SCF content much less than the estimated solubility limit.
Nitrogen comes out of solution much more quickly causing a more aggressive foaming reaction. This more aggressive reaction has both positive and negative aspects. On the positive side, nitrogen will tend to create a higher cell density which also translates to an overall smaller cell size. In addition nitrogen will create cell structure in thinner walls than will CO2. The images below show a clarified random copolymer PP molded in a container with a wall thickness of 0.7 mm. It can be seen that at 4% CO2, there is no visible cell structure in the wall of the container while at 1.5% nitrogen, the container has a cell structure that causes opacity. This occurs as the polymer freezes before the CO2 can cause the cells to grow while the nitrogen rapids expands the cells.
Photo 1:

4% CO2 at 0.68 mm
2% N2 at 0.68 mm
The negative aspect of the aggressive foaming seen with nitrogen is that nitrogen will typically produce a more pronounce surface change than CO2.







